Scientific Images
Scroll over each picture to learn more
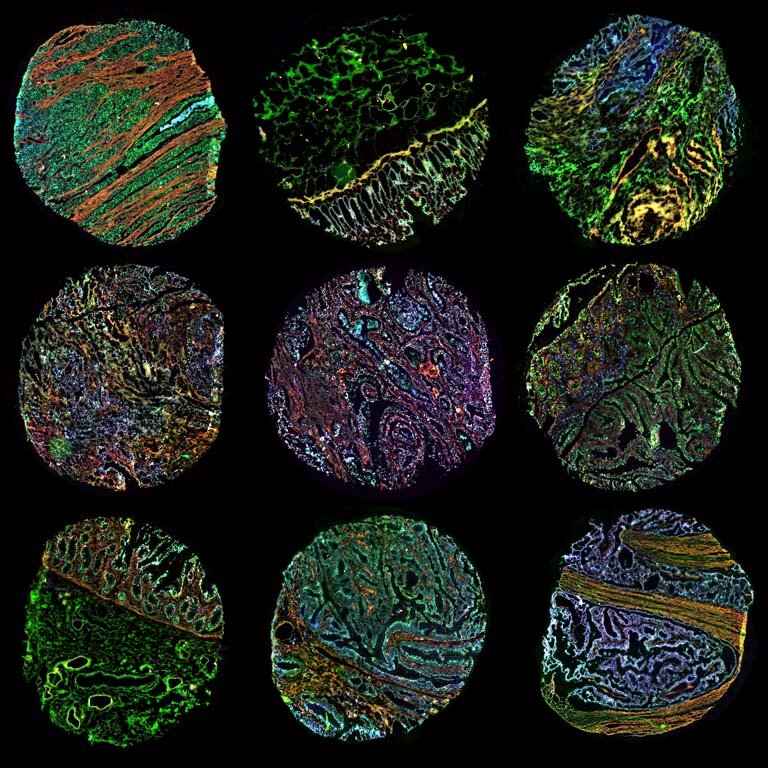
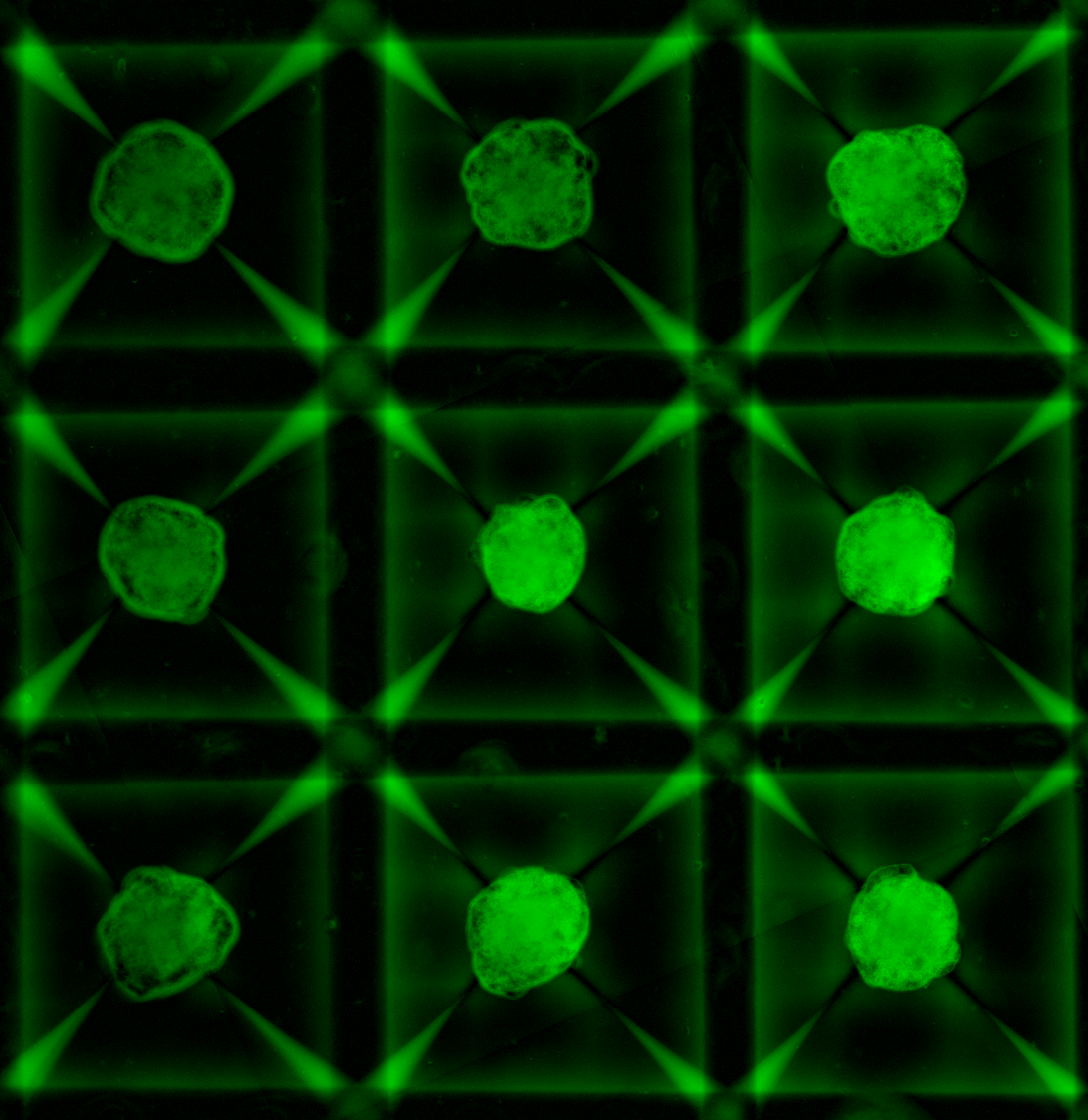
Extracellular Matrix: Microarray Views Nathan E. Reticker-Flynn, David F. Braga Malta, Sangeeta Bhatia Koch Institute Image Awards Winner 2014 Extracellular matrix (ECM) molecules are a family of molecules that fill the space around cells. During cancer progression, interactions between cells and their surrounding ECM are perturbed in a manner that permits invasion and metastasis. To understand this process better, we engineered a microarray technology that allows us to query interactions between cells and hundreds of unique combinations of ECM molecules. This image depicts the ECM microarray stained for all of the different ECM combinations, presented as small islands, present on the arrays.
Cellular Mandala: A Microkaleiodoscope of Bioengineering Justin Voog, MD, PhD; Neal Peterson, MFA; Sangeeta Bhatia, MD, PhD Koch Institute Image Awards Winner 2018 This collage of micrographs highlights the complex and complementary interactions between liver cells (hepatocytes) and supportive stromal cells. Within this image we are able to visualize fluorescently labeled proteins produced by stromal cells that are important for hepatocytes grown in the laboratory. This image is an example of the biologic beauty that can be observed (and created) when science and engineering are coupled. A major goal of our work is to develop in vitro liver models to better understand regenerative cues that are exploited in liver cancer and metastasis. This set of experiments identified the cellular distribution and expression of stromal factors (and potential therapeutic targets) necessary for maintaining liver models in the lab.
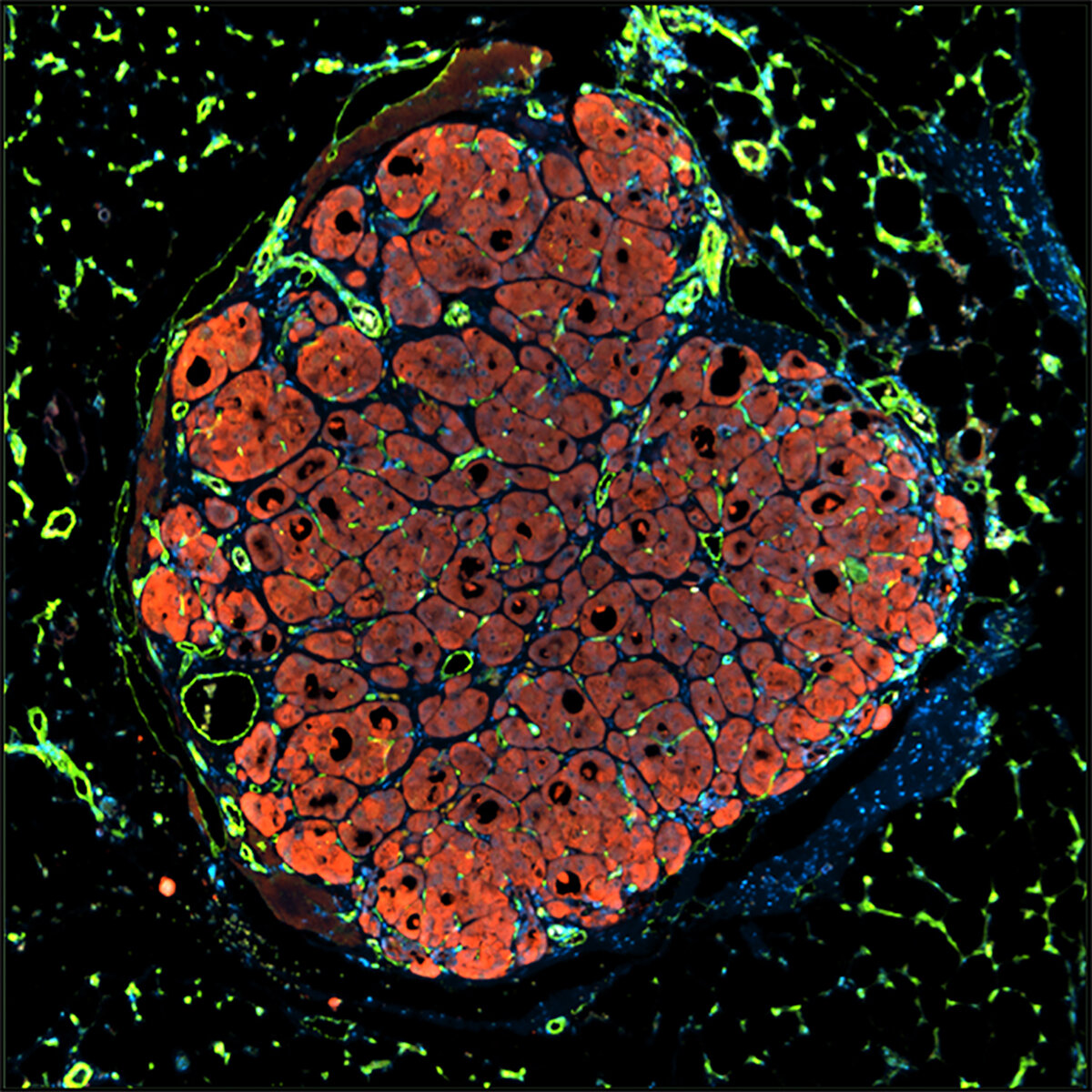
Blood Vessel Architecture in a Breast Tumor Alex Bagley, Sangeeta Bhatia Koch Institute Image Awards Winner 2012 This image was taken in a live subject, and depicts blood vessel architecture in a sample of human breast cancer grown in an animal host (called a xenograft). We were quantitatively comparing differences in tumor microvascular architecture as a function of tumor type and site of implantation. Here, we discovered that breast xenografts display a more complex and extensive vascular network than ovarian tumor nodules in the peritoneal cavity.
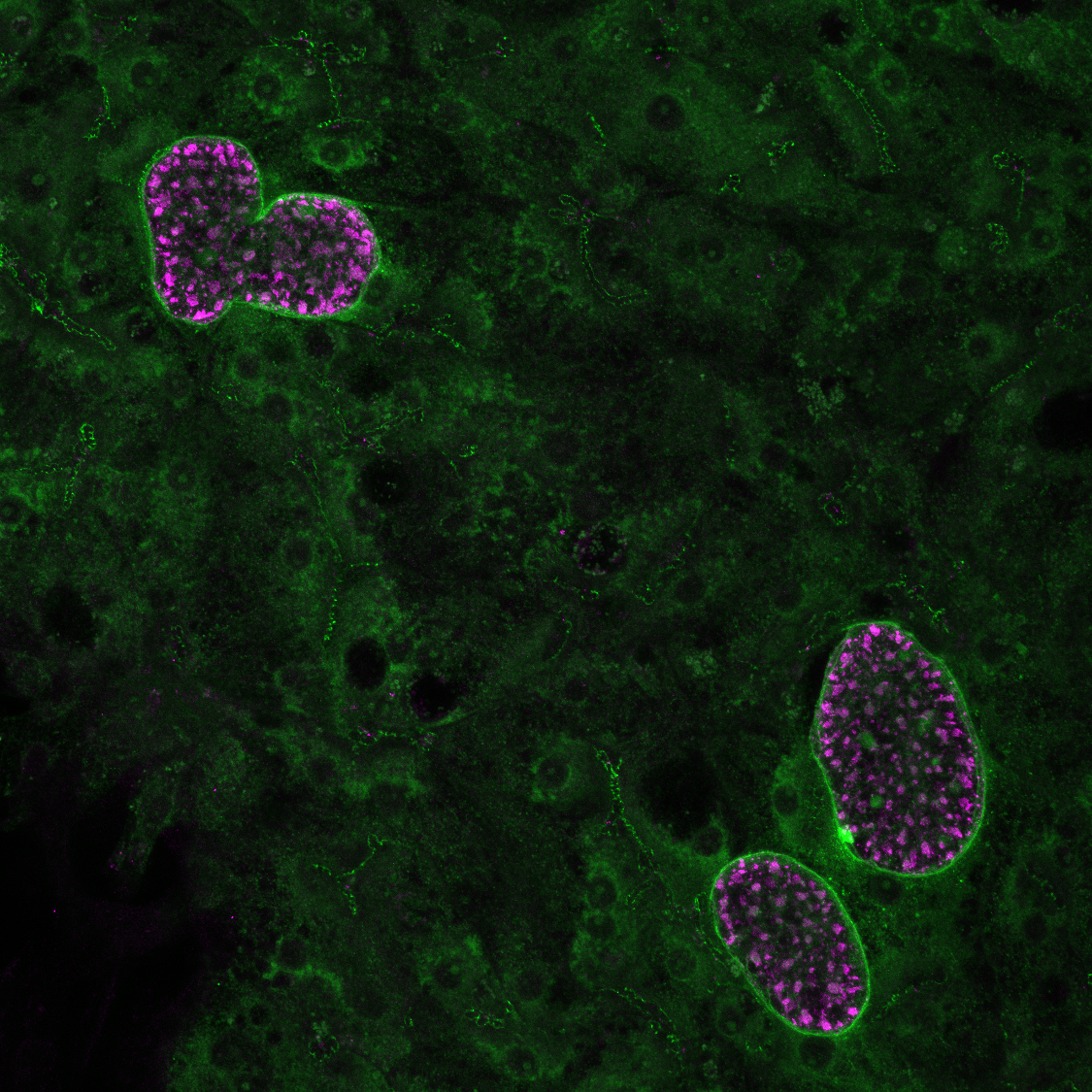
Nanosensors for Personalized Medicine Jaideep S. Dudani, Ester J. Kwon, Sangeeta N. Bhatia Koch Institute Image Awards Winner 2017 The character of tumors differs greatly from person to person. When deciding what treatment to give a patient, access to more information about the tumor’s behavior can help a clinical team pick the therapy that has the best chance of being effective. We built a tool, called a nanosensor, which can be applied to thin slices of a tumor biopsy sample to tell us about some of the behaviors of that individual cancer. Our nanosensor technology combines two individual functions: first it finds and sticks to receptors that are present on tumor cells, and if those tumor cells produce unusual levels of an active protein, called an enzyme, the nanosensor releases a signal. This image shows a collection of thin slices of colon cancer tissue from different patients. To these biopsies, we added our nanosensor, which is marked in green. The cancer cell receptor that serves as the target for the nanosensor is marked in red, and the enzyme is shown in cyan. The different samples show very different patterns of expression, which is a visual reminder that not all tumors are the same – and even individual tumors vary from place to place. We are working on using these nanosensors to determine which medicines might the best match for each tumor pattern.
In Robotico Model of Nanoparticle Tissue Penetration, Image C Sangeeta Bhatia, Radhika Nagpal, Sabine Hauert, and Michael Rubenstein Koch Institute Image Awards Winner 2013 Understanding how nanoparticles distribute in cancer tissue is important to predict if they will be able to reach and treat all tumor cells. Because nanoparticles are difficult to visualize, we use robots as a way to model and brainstorm about the dynamics involved. In this image, nanoparticle-robots shown in green leave a virtual vessel to diffuse through tissue and bind to cell-robots (red) using wireless communication. Nanoparticle-robots can then unbind or internalize in the cell (purple) where they deliver their treatment. Kilobot robots developed by the Wyss Institute at Harvard were programmed as either nanoparticles-robots or cell-robots. Cell-robots were manually placed inside the virtual tissue and nanoparticle-robots were placed in a virtual vessel on the right side of the image. All robots were then activated and left to run fully autonomously. These images were taken four minutes after the beginning of the experiment.
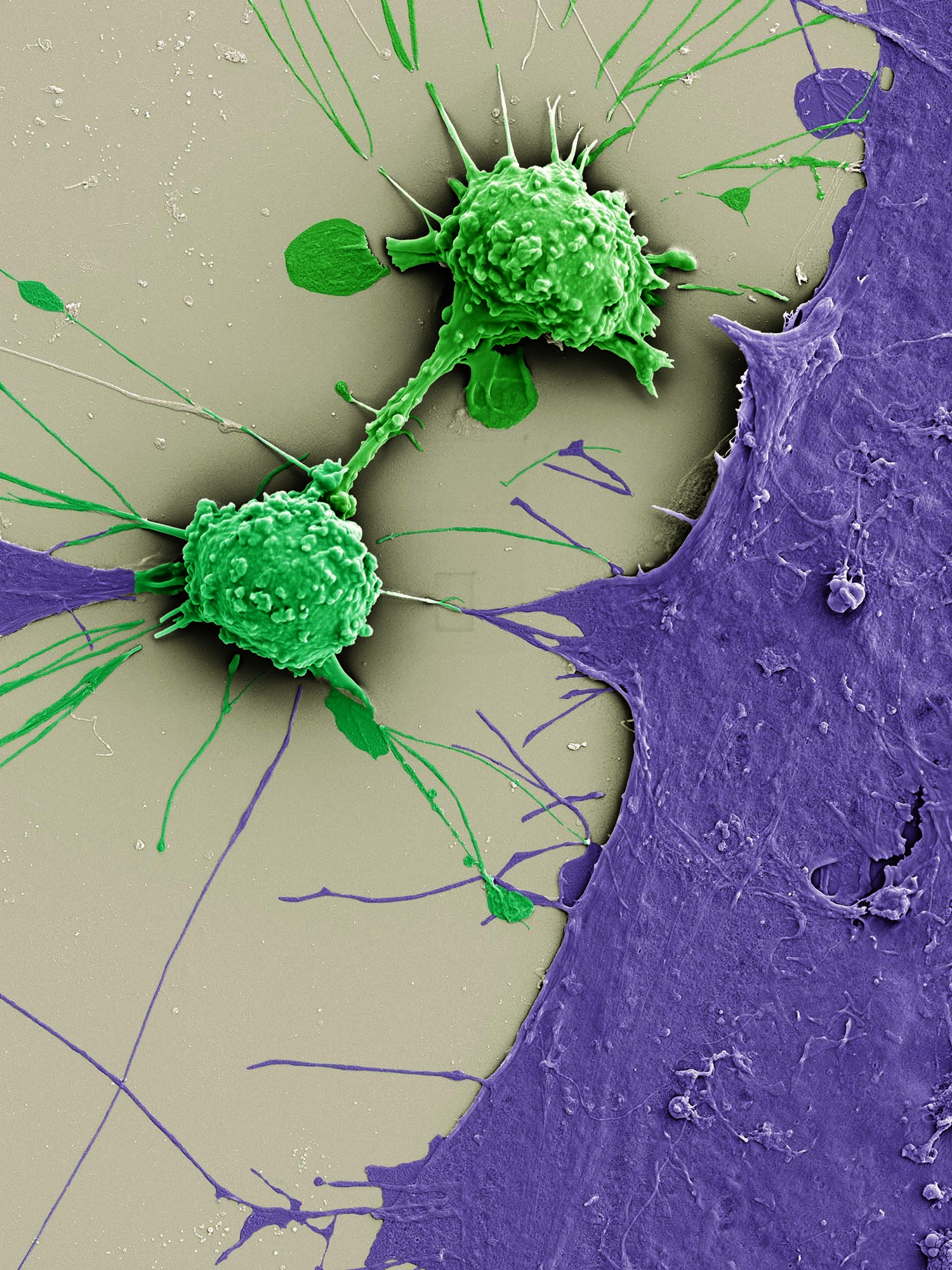
Natural Born Killers: Activating the Immune System to Fight Disease Allison Demas, David Mankus, Margaret Bisher, Abigail Lytton-Jean, Galit Alter, Sangeeta Bhatia Koch Institute Image Awards Winner 2019 Special operatives and frontline defenders against infection and disease, natural killer (NK) cells are the ninjas of the immune system. The Bhatia and Alter Labs seek to visualize the process of activation and attack. The NK cell seen here has been deposited on a glass slide alongside parasites and therapeutic antibodies. Preparing for battle, its surface transforms from smooth to bumpy and protrusions start to emerge. Malaria is the enemy this time, but similar approaches are also being tested against cancer.
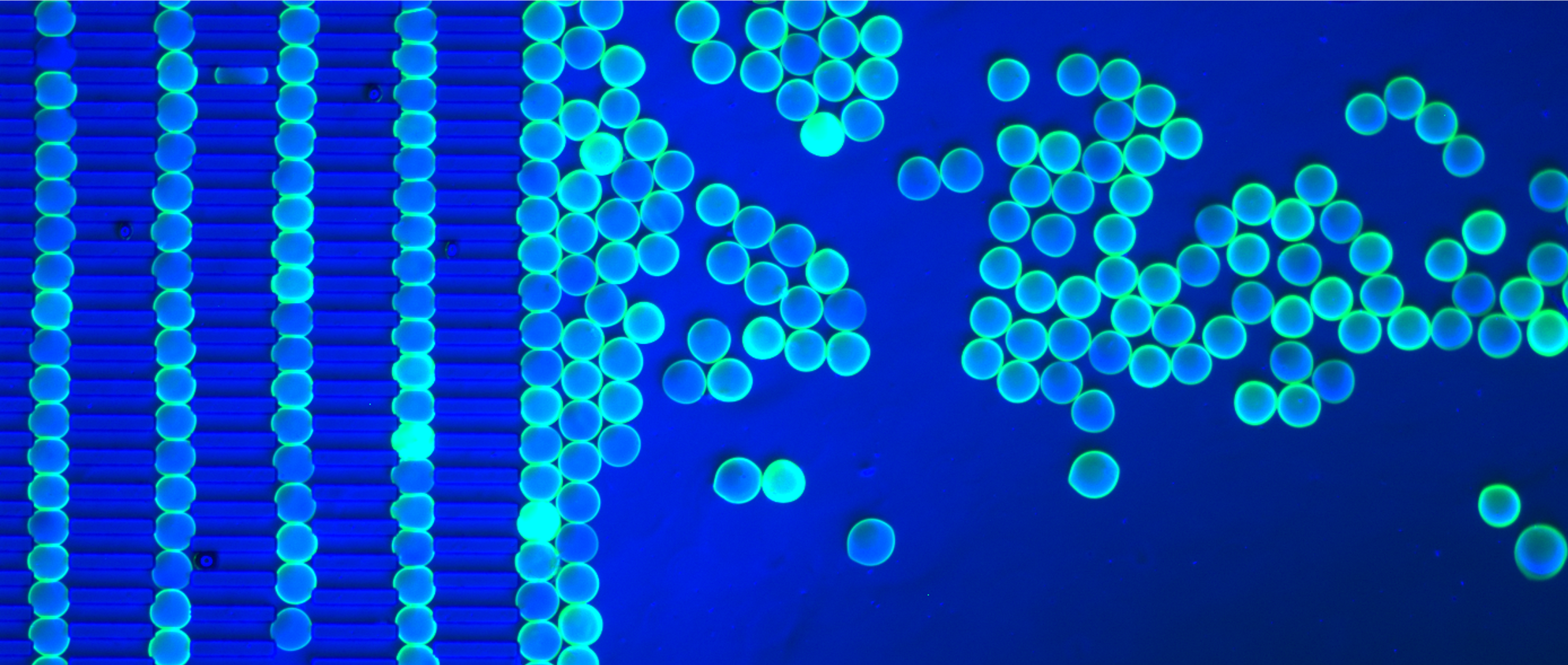
Pillars and Polymers Sangeeta Bhatia and Cheri Li Koch Institute Image Awards Winner 2013 This image shows the inside of a microfluidic chamber containing a line of pillars that are trapping first a layer of hard polystyrene spheres, and then the chamber is filled with model hydrogel spheres (green). I took this image to see whether the hydrogels were able to be retained in this chamber, and how the stiffness of the hydrogels affected the manner of the packing (you can see that they squish together a bit so that they are no longer perfectly round). Packed chambers such as this are analogous to packed-bed catalyst reactors used in much of chemical processing. Our goal is to encapsulate liver cells within these hydrogel spheres. Liver cells are often called the chemical processing plants of the body: by packing 'catalytic' spheres of hydrogels containing liver cells, we could then perfuse drugs through the chamber and study the effect that the liver cells have on the drugs.
A mixture of hepatocyte pucks and fibroblasts are encapsulated using a (B) microfluidic droplet generating device to polymerize ∼100 μm-diameter spherical cell-laden hydrogels. From our paper "Micropatterned Cell–Cell Interactions Enable Functional Encapsulation of Primary Hepatocytes in Hydrogel Microtissues"
Cancers can be very difficult to detect, even using sophisticated imaging techniques. Physicians often use biomarkers (proteins, DNA, or other things made by the body) in blood or urine to detect cancer, but many cancers do not produce biomarkers in high enough quantities, or even at all. Our team has designed nanoparticle-based 'synthetic biomarkers' that are injected, interact with the tumor, and create a signal in the urine. Using a test strip literally made of paper that works like a home pregnancy test and costs about 25 cents, we can detect these urinary biomarkers and diagnose cancer inexpensively and anywhere in the world. These images demonstrate how 'frugal engineering' can combine low-cost materials with sophisticated nanoscale techniques to enable inexpensive, point-of-care diagnosis of diseases that otherwise must be identified using costly and skill-intensive techniques that are unavailable in places like the developing world. Image by Bryce Vickmark
Anti-Bacterial Nanoparticles Ester J. Kwon, Matt Skalak, Sangeeta Bhatia Koch Institute Image Awards Winner 2017 Bacterial infections are an important public health concern. We are designing new antibiotics, and testing whether we can deliver them into the lung to fight pneumonia caused by bacteria. We stuffed anti-bacterial peptides into tiny packages called nanoparticles and looked to see if they can spread throughout the lung in an animal model. This image shows a slice through a mouse lung that was infected with bacteria and treated with our therapeutic peptide-nanoparticles. The red color marks the bacteria and the green color marks our therapeutic nanoparticles. We also marked the cells of the lung in blue and cells that form part of the body’s own immune response to the bacteria in magenta. When we take a view of the whole lung, we can see our particles escape the large airway “highways” (large holes) and spread through the lung where they can fight the infection.
Artwork by a previous postdoc, Tal Danino.
Human hepatocyte-like cells derived from iPS cells, with additional help of small molecules identified via high-throughput screening.
Pillars and Polymers, Image #2 Sangeeta Bhatia and Cheri Li Koch Institute Image Awards Winner 2013 This image shows the inside of a microfluidic chamber containing a line of pillars that are trapping first a layer of hard polystyrene spheres, and then the chamber is filled with model hydrogel spheres (teal). I took this image to see whether the hydrogels were able to be trapped in a regular array in this chamber. Packed chambers such as this are analogous to packed-bed catalyst reactors used in much of chemical processing. Our goal is to encapsulate liver cells within these hydrogel spheres. Liver cells are often called the chemical processing plants of the body: by packing 'catalytic' spheres of hydrogels containing liver cells, we could then perfuse drugs through the chamber and study the effect that the liver cells have on the drugs.
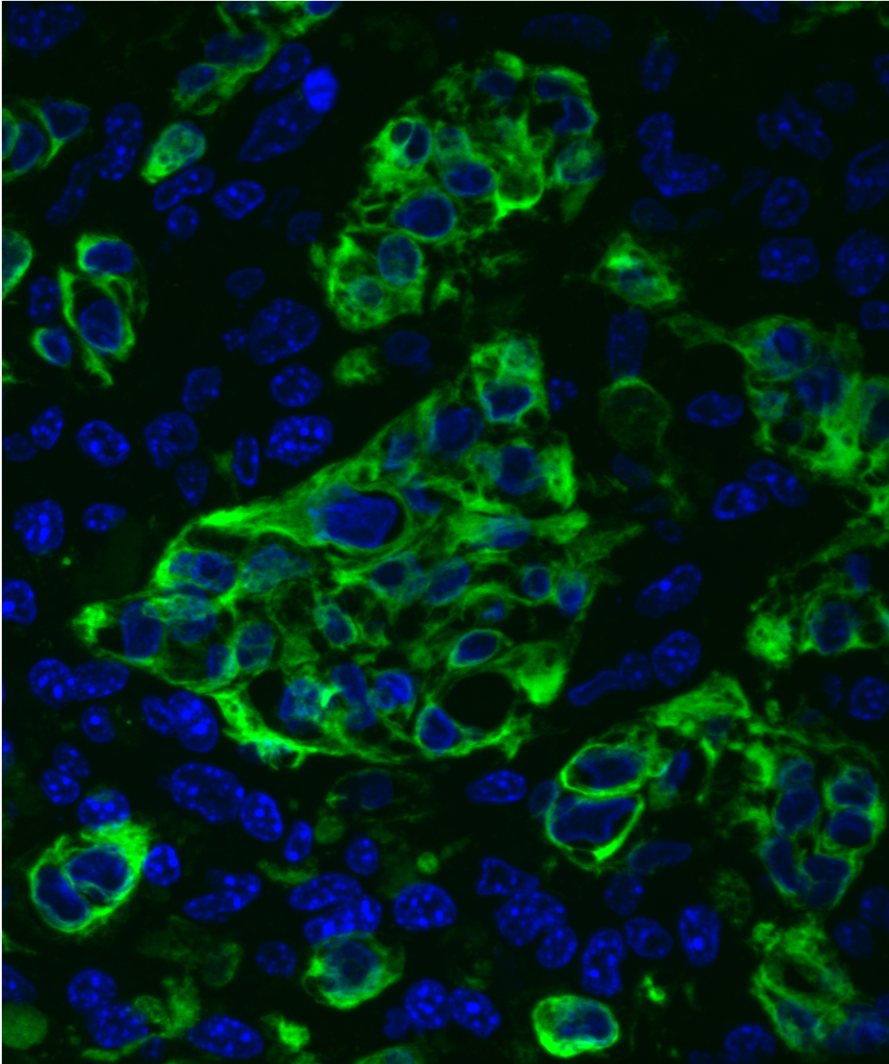
Tiny Trojan Horses: Tumor-Penetrating Nanoparticles Infiltrate Cancer Cells Liangliang Hao, Srivatsan Raghavan, Emilia Pulver, Jeffrey Wyckoff, Sangeeta Bhatia Koch Institute Image Awards Winner 2017 You can lead a nanoparticle to tumor cells, but you can’t make them shrink—at least not until the particle gets inside. This image shows biocompatible nanoparticles (yellow) inside clusters of pancreatic cancer cells (pink). The particles’ two-peptide uptake system—one to target the tumor, the second to penetrate it—was specially designed to overcome known difficulties in treating pancreatic cancer, but the Bhatia Lab hopes to expand the use of this modular delivery system for other cancer types as well.
Launching a Satellite Liver: Tissue Engineering in Action Chelsea Fortin, Sangeeta Bhatia Koch Institute Image Awards Winner 2016 The liver is known for its regenerative properties, but certain types of damage are irreversible. To combat the growing shortage of replacement organs, researchers have grown liver cells on a specially patterned matrix of blood vessels (green) and transplanted them into their disease model. This image shows how, in response to damage in the original organ, the cells (orange) reorganize and expand, integrating blood (white) from the host to support their growth. The creation of such "satellite livers" could greatly improve outcomes for patients suffering from liver disease, cirrhosis, and liver cancer.
Cloak and Swagger: Learning From the Enemy Arnav Chhabra, David Mankus, Margaret Bisher, Abigail Lytton-Jean, Sangeeta Bhatia Koch Institute Image Awards Winner 2020 When someone receives an organ transplant, their own immune cells may identify it as a foreign body and try to destroy it. The Bhatia Lab is stealing one of cancer’s most subversive weapons—its ability to elude detection by the immune system—to cast an invisibility cloak over donated cells. By reprogramming them to express the same surface markers as cancer cells, researchers hide transplanted cells (purple) from would-be aggressors (green) in the body. Although superficial, these changes to the cells’ appearance could have deep therapeutic benefit.
Tiny Trojan Horses 2 Liangliang Hao, Srivatsan Raghavan, Emilia Pulver and Sangeeta Bhatia Koch Institute Image Awards Winner 2020 Pancreatic cancer is the only major cancer with a five-year relative survival rate in the single digits, in part because the desmoplastic stroma prevents drugs from reaching the pancreas. To help overcome that obstacle, we engineered nanoparticles that can penetrate deeply to attack the tumor cells specifically. The fluorescent nanoparticles are penetrating into three-dimensional spherical cluster of tumor cells called organoids mimicking the complex cellular component in real tumors. In this image, blue coloring shows the cell nuclei, green the cell membrane, and magenta the nanoparticles.
These are images of the microlivers (HEALs) that can be implanted in animals. The bottom ones are a first generation construct. The top example shows how a new generation method for making microlivers can also be scaled up to one day make much larger implants. A. Chen, Thomas, D. K., Ong, L. L., Schwartz, R. E., Golub, T. R., and Bhatia, S. N.,“Humanized mice with ectopic artificial liver tissues.”, Proc Natl Acad Sci USA, vol. 108, no. 29, pp. 11842-7, 2011. K. R. Stevens, Ungrin, M. D., Schwartz, R. E., Ng, S., Carvalho, B., Christine, K. S., Chaturvedi, R. R., Li, C. Y., Zandstra, P. W., Chen, C. S., and Bhatia, S. N.,“InVERT molding for scalable control of tissue microarchitecture.”, Nat Commun, vol. 4, p. 1847, 2013.
These are images of the microlivers (HEALs) that can be implanted in animals. The bottom ones are a first generation construct. The top example shows how a new generation method for making microlivers can also be scaled up to one day make much larger implants. A. Chen, Thomas, D. K., Ong, L. L., Schwartz, R. E., Golub, T. R., and Bhatia, S. N.,“Humanized mice with ectopic artificial liver tissues.”, Proc Natl Acad Sci USA, vol. 108, no. 29, pp. 11842-7, 2011. K. R. Stevens, Ungrin, M. D., Schwartz, R. E., Ng, S., Carvalho, B., Christine, K. S., Chaturvedi, R. R., Li, C. Y., Zandstra, P. W., Chen, C. S., and Bhatia, S. N.,“InVERT molding for scalable control of tissue microarchitecture.”, Nat Commun, vol. 4, p. 1847, 2013.
Engineering a perfusable 3D human liver platform from iPS cells Schepers et al, 2013
After plasma treatment, seeded primary human hepatocytes selectively attach to the remaining collagen pattern and are subsequently surrounded by supportive stromal cells. Read more: https://www.sciencedirect.com/science/article/pii/S1931312818300374?via%3Dihub
Artificially engineered tissues (e.g., liver) could serve as organ-substitutes for clinical transplantation, but to date most engineered tissue has been functionally limited by inadequate blood supply. Here, we used microfabrication technology to build engineered tissue composed of hepatocytes (red) interlaced with an endothelial vascular lattice (green, “blood vessels”). By Kelly Stevens
Blood, Heat, and Tumors: Improving Drug Delivery with Gold Nanorods Alex Bagley, Sangeeta Bhatia Koch Institute Image Awards Winner 2014 Blood vessels are highways through the body. They can transport drugs to cancer cells, but finding the appropriate ramp to exit the vessel can be tricky. This image shows a network of blood vessels (green) and collagen (purple) infused with gold nanorods (yellow) inside a living tumor. When researchers heat the particles with near-infrared light, the blood vessels become leaky, making it easier to deliver a therapeutic cargo to its final destination. Because blood vessels provide a universal transport system, such combination therapy has widespread implications for treatment, regardless of cancer type or specific drug needed.
Cell Patterns in An Engineered Tissue, Image #1 Sangeeta Bhatia and Kelly Stevens Koch Institute Image Awards Winner 2013 To build engineered tissues that will ultimately be useful as implantable human therapies (i.e., for organ repair or replacement) we must have complete understanding of the ‘design specifications’ on how tissues are (in the body) and can be constructed so that they are most functional. We used microfabrication technologies to pattern several types of cells (endothelial cells, green; fibroblasts, red) in a three-dimensional engineered tissue. We are trying to understand how different cell types communicate with one another as they come together to form tissues. By developing technologies to alter spatial relationships and organization between the different cell types we can study how organization impacts cell behavior and tissue function.
Cell-laden hydrogel microtissues aggregated by cell adhesion.
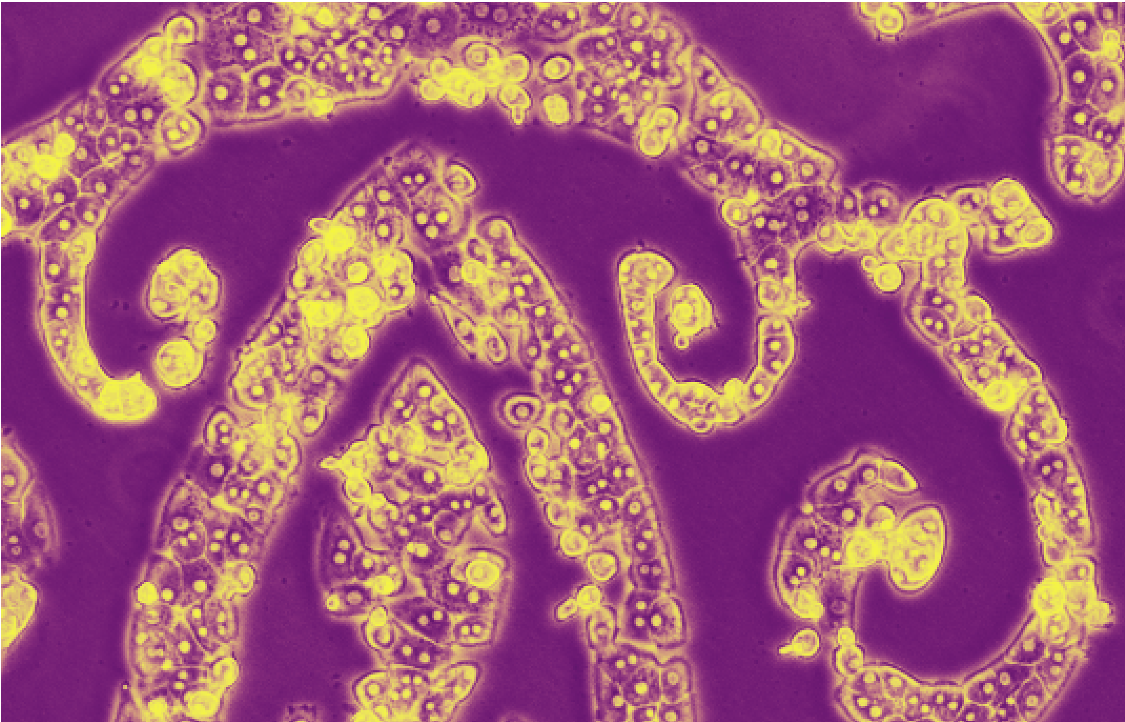
Confocal Microscopy Image of Organoids of Induced Pluripotent Stem Cell derived Hepatocytes and Fibroblasts By Robert Schwartz and Kelly Stevens
Cell-laden hydrogel microtissues aggregated by cell adhesion. Blue – nuclei, red – actin.
Artificially engineered tissues (e.g., liver) could serve as organ-substitutes for clinical transplantation, but to date most engineered tissue has been functionally limited by inadequate blood supply. Here, we used microfabrication technology to build engineered tissue composed of hepatocytes (red) interlaced with endothelial vascular cords (green, “blood vessels”). Image by Kelly Stevens
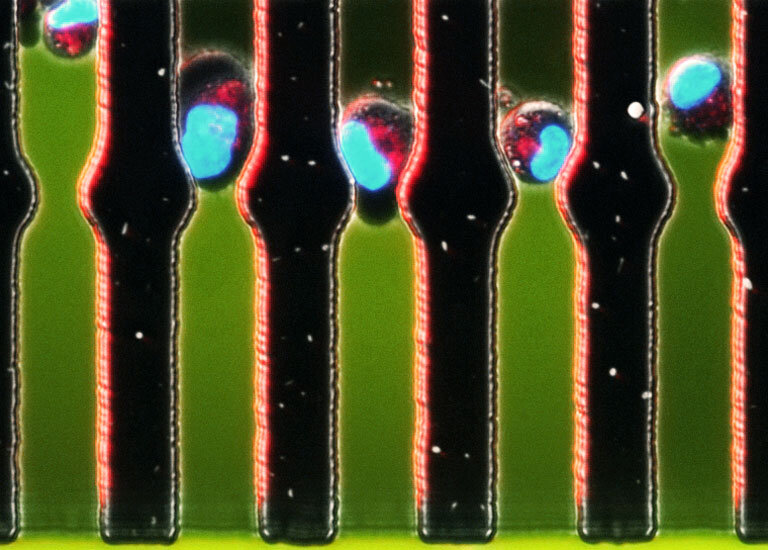
Motor Control: How Does a Cancer Cell Power its Movement? Salil Desai, Sangeeta Bhatia Koch Institute Image Awards Winner 2012 Much of the deadly impact of cancer is due to metastasis, the spread of cancer cells to distant tissues. But what drives a cancer cell’s movementthrough the body? Here, researchers investigate the role of mitochondria, the “powerhouses” inside cancer cells, as the cells travel through tiny fluid-filled channels.
Read more: L. Mancio-Silva, H. E. Fleming, A. B. Miller, S. Milstein, A. Liebow, P. Haslett, L. Sepp-Lorenzino, S. N. Bhatia, Improving drug discovery by nucleic acid delivery in engineered human microlivers. Cell Metab. 29, 727-735.e3 (2019).
Read more: N. Gural, L. Mancio-Silva, A. B. Miller, A. Galstian, V. L. Butty, S. S. Levine, R. Patrapuvich, S. P. Desai, S. A. Mikolajczak, S. H. I. Kappe, H. E. Fleming, S. March, J. Sattabongkot, S. N. Bhatia, In vitro culture, drug sensitivity, and transcriptome of plasmodium vivax hypnozoites. Cell Host Microbe. 23, 395-406.e4 (2018).
P. vivax EEFs from MPCC cultures Read more: N. Gural, L. Mancio-Silva, A. B. Miller, A. Galstian, V. L. Butty, S. S. Levine, R. Patrapuvich, S. P. Desai, S. A. Mikolajczak, S. H. I. Kappe, H. E. Fleming, S. March, J. Sattabongkot, S. N. Bhatia, In vitro culture, drug sensitivity, and transcriptome of plasmodium vivax hypnozoites. Cell Host Microbe. 23, 395-406.e4 (2018).
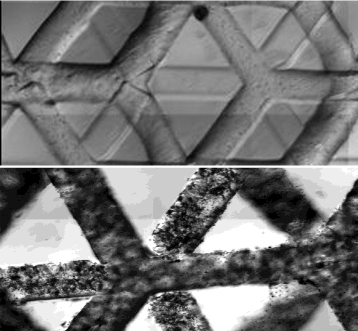
A multiscale carbohydrate-glass lattice. Multilayered lattices are fabricated in minutes with precise lateral and axial positioning resolution. Read more: J. S. Miller, K. R. Stevens, M. T. Yang, B. M. Baker, D.-H. T. Nguyen, D. M. Cohen, E. Toro, A. A. Chen, P. A. Galie, X. Yu, R. Chaturvedi, S. N. Bhatia, C. S. Chen, Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).